Reaction data
 +
+ 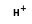 =
= 
Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.)
Go To: Top, References, Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Data compiled by: John E. Bartmess
| ΔrH° (kJ/mol) | Method | Reference | Comment |
|---|---|---|---|
| 1456. ± 4.2 | CIDC | Angel and Ervin, 2006 | gas phase; B |
| 1467.7 ± 3.7 | D-EA | Gunion, Gilles, et al., 1992 | gas phase; Derived BDE from D-EA cycle: 87.6±2.2 kcal/mol; B |
| 1461. ± 8.8 | G+TS | Bartmess, Scott, et al., 1979 | gas phase; Shiner, Vorner, et al., 1986: tautomer acidities ΔHacid(ortho) = 343.9±3.1 kcal, para = 340.1±2 kcal. However, Capponi, Gut, et al., 1999 based on aq. soln. results, imply 18 and 14 kcal/mol difference.; value altered from reference due to change in acidity scale; B |
| 1454. ± 7.9 | CIDC | Angel and Ervin, 2004 | gas phase; B |
| 1466. ± 13. | Endo | DeTuri and Ervin, 1998 | gas phase; Re-analyzed (private comm., KME): acidity strengthened by 1.2 kcal/mol from value in paper. BDE now 88.7 kcal/mol; B |
| 1466. ± 9.6 | G+TS | Cumming and Kebarle, 1978 | gas phase; B |
| >1457. ± 7.1 | D-EA | Richardson, Stephenson, et al., 1975 | gas phase; B; Data excluded from overall average |
References
Go To: Top, Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.), Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Angel and Ervin, 2006
Angel, L.A.; Ervin, K.M.,
Gas-phase acidities and O-H bond dissociation enthalpies of phenol, 3-methylphenol, 2,4,6-trimethylphenol, and ethanoic acid,
J. Phys. Chem. A, 2006, 110, 35, 10392-10403, https://doi.org/10.1021/jp0627426
. [all data]
Gunion, Gilles, et al., 1992
Gunion, R.F.; Gilles, M.K.; Polak, M.L.; Lineberger, W.C.,
Ultraviolet Photoelectron Spectroscopy of the Phenide, Benzyl, and Phenoxide Anions.,
Int. J. Mass Spectrom. Ion Proc., 1992, 117, 601, https://doi.org/10.1016/0168-1176(92)80115-H
. [all data]
Bartmess, Scott, et al., 1979
Bartmess, J.E.; Scott, J.A.; McIver, R.T., Jr.,
The gas phase acidity scale from methanol to phenol,
J. Am. Chem. Soc., 1979, 101, 6047. [all data]
Shiner, Vorner, et al., 1986
Shiner, C.S.; Vorner, P.E.; Kass, S.R.,
Gas phase acidities and heats of formation of 2,4- and 2,5- cyclohexadien-1-one, the keto tautomers of phenol,
J. Am. Chem. Soc., 1986, 108, 5699. [all data]
Capponi, Gut, et al., 1999
Capponi, M.; Gut, I.G.; Hellrung, B.; Persy, G.; Wirz, J.,
Ketonization equilibria of phenol in aqueous solution,
Can. J. Chem., 1999, 77, 5-6, 605-613, https://doi.org/10.1139/v99-048
. [all data]
Angel and Ervin, 2004
Angel, L.A.; Ervin, K.M.,
Competitive threshold collision-induced dissociation: Gas-phase acidity and O-H bond dissociation enthalpy of phenol,
J. Phys. Chem. A, 2004, 108, 40, 8346-8352, https://doi.org/10.1021/jp0474529
. [all data]
DeTuri and Ervin, 1998
DeTuri, V.F.; Ervin, K.M.,
Proton transfer between Cl- and C6H5OH. O-H bond energy of phenol,
Int. J. Mass Spectrom., 1998, 175, 1-2, 123-132, https://doi.org/10.1016/S0168-1176(98)00112-8
. [all data]
Cumming and Kebarle, 1978
Cumming, J.B.; Kebarle, P.,
Summary of gas phase measurements involving acids AH. Entropy changes in proton transfer reactions involving negative ions. Bond dissociation energies D(A-H) and electron affinities EA(A),
Can. J. Chem., 1978, 56, 1. [all data]
Richardson, Stephenson, et al., 1975
Richardson, J.H.; Stephenson, L.M.; Brauman, J.I.,
Photodetachment of electrons from phenoxides and thiophenoxide,
J. Am. Chem. Soc., 1975, 97, 2967. [all data]
Notes
Go To: Top, Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.), References
- Symbols used in this document:
ΔrH° Enthalpy of reaction at standard conditions - Data from NIST Standard Reference Database 69: NIST Chemistry WebBook
- The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment. However, NIST makes no warranties to that effect, and NIST shall not be liable for any damage that may result from errors or omissions in the Database.
- Customer support for NIST Standard Reference Data products.