Reaction data
Ar+ + 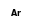 = (Ar+ •
= (Ar+ • 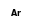 )
)
Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.)
Go To: Top, References, Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Data compiled by: Michael M. Meot-Ner (Mautner) and Sharon G. Lias
| ΔrH° (kcal/mol) | Method | Reference | Comment |
|---|---|---|---|
| 29.3 | PI | Dehmer and Pratt, 1982 | gas phase; M |
| 2.3 ± 0.5 | PES | Dehmer and Dehmer, 1978 | gas phase; Ar2+[II(1/2u)]; M |
| 28.4 | PI | Ng, Trevor, et al., 1977 | gas phase; M |
| 28.8 | SCATTERING | Lorentz, Olson, et al., 1973 | gas phase; M |
| 27.8 | PHPMS | Teng and Conway, 1973 | gas phase; switching reaction(N2+)Ar; Turner and Conway, 1979, Liu and Conway, 1975; M |
| 30.7 | PDiss | Moseley, Saxon, et al., 1977 | gas phase; M; Data excluded from overall average |
| 30.9 | SCATTERING | Mittman and Weise, 1974 | gas phase; M; Data excluded from overall average |
References
Go To: Top, Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.), Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Dehmer and Pratt, 1982
Dehmer, P.M.; Pratt, S.T.,
Photoionization of ArKr, ArXe, and KrXe and bond dissociation energies of the rare gas dimer ions,
J. Chem. Phys., 1982, 77, 4804. [all data]
Dehmer and Dehmer, 1978
Dehmer, P.M.; Dehmer, J.L.,
Photoelectron spectra of Ar2 and Kr2 and dissociation energies of the rate gas dimer ions,
J. Chem. Phys., 1978, 69, 125. [all data]
Ng, Trevor, et al., 1977
Ng, C.Y.; Trevor, D.J.; Mahan, B.H.; Lee, Y.T.,
Photoionization Studies of the Kr2 and Ar2 van der Vaals Molecules,
J. Chem. Phys., 1977, 66, 2, 446, https://doi.org/10.1063/1.433989
. [all data]
Lorentz, Olson, et al., 1973
Lorentz, D.C.; Olson, R.E.; Conklin, G.M.,
Rainbow Scattering for Ar+ + Ar and Xe+ + Xe,
Chem. Phys. Lett., 1973, 20, 6, 589, https://doi.org/10.1016/0009-2614(73)80508-1
. [all data]
Teng and Conway, 1973
Teng, H.H.; Conway, D.C.,
Ion - Molecule Equilibria in Mixtures of N2 and Ar,
J. Chem. Phys., 1973, 59, 5, 2316, https://doi.org/10.1063/1.1680338
. [all data]
Turner and Conway, 1979
Turner, D.L.; Conway, D.C.,
Study of the 2Ar + Ar2+ = Ar + Ar3+ Reaction,
J. Chem. Phys., 1979, 71, 4, 1899, https://doi.org/10.1063/1.438544
. [all data]
Liu and Conway, 1975
Liu, W.F.; Conway, D.C.,
Ion - Molecule Reactions in Ar at 296, 195, and 77 K,
J. Chem. Phys., 1975, 62, 8, 3070, https://doi.org/10.1063/1.430906
. [all data]
Moseley, Saxon, et al., 1977
Moseley, J.T.; Saxon, R.P.; Huber, B.A.; Cosby, P.C.; Abouaf, R.; Tadjeddine, M.,
Photofragment spectroscopy and potential curves of Ar2+,
J. Chem. Phys., 1977, 67, 1659. [all data]
Mittman and Weise, 1974
Mittman, H.U.; Weise, H.P.,
Scattering of Ions V. Elastic Scattering of the Symmetric Rare Gas Ion - Rare Gas Atom Systems,
Z. Naturforsch., 1974, A29, 400. [all data]
Notes
Go To: Top, Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.), References
- Symbols used in this document:
ΔrH° Enthalpy of reaction at standard conditions - Data from NIST Standard Reference Database 69: NIST Chemistry WebBook
- The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment. However, NIST makes no warranties to that effect, and NIST shall not be liable for any damage that may result from errors or omissions in the Database.
- Customer support for NIST Standard Reference Data products.