Reaction data
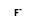 +
+ 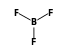 = (
= (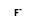 •
•  )
)
Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.)
Go To: Top, References, Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Data compiled as indicated in comments:
B - John E. Bartmess
M - Michael M. Meot-Ner (Mautner) and Sharon G. Lias
| ΔrH° (kJ/mol) | Method | Reference | Comment |
|---|---|---|---|
| 331. ± 8.4 | TDEq | Veljkovic, Neskovic, et al., 1991 | gas phase; Fluoride Affinity: 37.5 kcal/mol less than AlF3; value altered from reference due to conversion from electron convention to ion convention; B |
| 328. ± 14. | TDAs | Aleshina, Borshchevskii, et al., 1996 | gas phase; value altered from reference due to conversion from electron convention to ion convention; B |
| 330. ± 40. | TDEq | Pyatenko, Guasarov, et al., 1984 | gas phase; value altered from reference due to conversion from electron convention to ion convention; B |
| 301. ± 21. | IMRE | Larson and McMahon, 1985 | gas phase; These relative affinities are ca. 10 kcal/mol weaker than threshold values (see Wenthold and Squires, 1995) for donors greater than ca. 27 kcal/mol in free energy. This discrepancy has not yet been resolved, though the stronger value appears preferable.; B,M |
| 385. ± 25. | Ther | Mallouk, Rosenthal, et al., 1984 | gas phase; Fluoride affinities from this method appear to be consistently about 10 kcal/mol too bound; B |
| 393. ± 21. | Ther | Krivtsov, Titova, et al., 1977 | gas phase; Halide affinities from this reference are too high. G3MP2B3 calculations indicate an dHaff ca. 46 kcal/mol; value altered from reference due to conversion from electron convention to ion convention; B |
References
Go To: Top, Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.), Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Veljkovic, Neskovic, et al., 1991
Veljkovic, M.; Neskovic, O.; Zmbov, K.F.; Borshchevsky, A.Y.; Vaisberg, V.E.; Sidorov, L.N.,
Heats of Formation of BF4- Ions and the Fluorine Anion Affinity of BF3 Molecules,
Rapid Commun. Mass Spectrom., 1991, 5, 1, 37, https://doi.org/10.1002/rcm.1290050111
. [all data]
Aleshina, Borshchevskii, et al., 1996
Aleshina, V.E.; Borshchevskii, Ya.; Korobov, V.M.; Sidorov, L.N.,
The Enthalpy of Addition of the Fluorine Anion to the BF3 and PF5 Molecules,
Russ. J. Phys. Chem., 1996, 70, 1085. [all data]
Pyatenko, Guasarov, et al., 1984
Pyatenko, A.T.; Guasarov, A.V.; Gorokhov, L.N.,
Thermochemistry of Negative Ions in the U-F System,
Russ. J. Phys. Chem., 1984, 58, 1. [all data]
Larson and McMahon, 1985
Larson, J.W.; McMahon, T.B.,
Fluoride and chloride affinities of the main group oxides, fluorides, oxofluorides, and alkyls. Quantitative scales of lewis acidities from ICR halide exchange equilibria,
J. Am. Chem. Soc., 1985, 107, 766. [all data]
Wenthold and Squires, 1995
Wenthold, P.G.; Squires, R.R.,
Bond dissociation energies of F2(-) and HF2(-). A gas-phase experimental and G2 theoretical study,
J. Phys. Chem., 1995, 99, 7, 2002, https://doi.org/10.1021/j100007a034
. [all data]
Mallouk, Rosenthal, et al., 1984
Mallouk, T.E.; Rosenthal, G.L.; Muller, G.; Brusasco, R.; Bartlett, N.,
Fluoride ion affinities of GeF4 and BF4 from thermodynamic and structural data for (SF2)2GeF6, ClO2GeF5, and ClO2BF4,
Inorg. Chem., 1984, 23, 3167. [all data]
Krivtsov, Titova, et al., 1977
Krivtsov, N.V.; Titova, K.V.; Rosolovskii, V.Ya.,
Thermochemical study of complex borates,
Russ. J. Inorg. Chem., 1977, 22, 374. [all data]
Notes
Go To: Top, Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.), References
- Symbols used in this document:
ΔrH° Enthalpy of reaction at standard conditions - Data from NIST Standard Reference Database 69: NIST Chemistry WebBook
- The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment. However, NIST makes no warranties to that effect, and NIST shall not be liable for any damage that may result from errors or omissions in the Database.
- Customer support for NIST Standard Reference Data products.