Reaction data
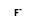 + F4U = (
+ F4U = (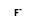 • F4U)
• F4U)
Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.)
Go To: Top, References, Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Data compiled by: John E. Bartmess
| ΔrH° (kJ/mol) | Method | Reference | Comment |
|---|---|---|---|
| 422. ± 15. | TDAs | Boltalina, Borshchevskii, et al., 1992 | gas phase; Critical review; value altered from reference due to conversion from electron convention to ion convention; B |
| 412. ± 16. | TDEq | Borshchevskii, Boltalina, et al., 1988 | gas phase; value altered from reference due to conversion from electron convention to ion convention; B |
| 427. ± 15. | TDEq | Nikitin, Igolkina, et al., 1986 | gas phase; Reanalyzed literature data, 14.5 kcal < AlF3; value altered from reference due to conversion from electron convention to ion convention; B |
| 424.26 | Ther | Pyatenko, Guasarov, et al., 1984 | gas phase; Critical review, other literature data corrected; value altered from reference due to conversion from electron convention to ion convention; B |
| 448. ± 36. | Ther | Pyatenko, Gusarov, et al., 1980 | gas phase; value altered from reference due to conversion from electron convention to ion convention; B |
| 410.0 ± 1.3 | TDEq | Sidorov, Skokan, et al., 1980 | gas phase; Fluoride Affinity: 22.2 kcal < AlF3; value altered from reference due to conversion from electron convention to ion convention; B |
References
Go To: Top, Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.), Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Boltalina, Borshchevskii, et al., 1992
Boltalina, O.V.; Borshchevskii, A.Y.; Sidorov, L.N.,
Thermochemistry of 3d Elements Fluorides and Their Negative Ions in Gas Phase,
Russ. J. Phys. Chem., 1992, 66, 1223. [all data]
Borshchevskii, Boltalina, et al., 1988
Borshchevskii, A.Ya.; Boltalina, O.V.; Sorokin, I.D.; Sidorov, L.N.,
Thermochemical Quantities for Gas Phase Iron, Uranium, and Molybdenum Fluorides, and Their Negative Ions.,
J. Chem. Thermodyn., 1988, 20, 5, 523, https://doi.org/10.1016/0021-9614(88)90080-8
. [all data]
Nikitin, Igolkina, et al., 1986
Nikitin, M.I.; Igolkina, N.A.; Skokan, E.V.; Sorokin, I.D.; Sidirov, L.N.,
Enthalpies of formation of the AlF4- ion,
J. Phys. Chem., 1986, 60, 22. [all data]
Pyatenko, Guasarov, et al., 1984
Pyatenko, A.T.; Guasarov, A.V.; Gorokhov, L.N.,
Thermochemistry of Negative Ions in the U-F System,
Russ. J. Phys. Chem., 1984, 58, 1. [all data]
Pyatenko, Gusarov, et al., 1980
Pyatenko, A.T.; Gusarov, A.V.; Gorokhov, L.N.,
Thermochemical Properties of Negative Ions in Vapor over UF4,
High Temp., 1980, 18, 857. [all data]
Sidorov, Skokan, et al., 1980
Sidorov, L.N.; Skokan, E.V.; Nitikin, M.I.; Sorokin, I.D.,
Mass spectrometric determination of enthalpies of dissociation of gaseous complex fluorides into neutral and charged particles. III. Heat of formation of UF5- and electron affinity of UF5,
Int. J. Mass Spectrom. Ion Phys., 1980, 35, 215. [all data]
Notes
Go To: Top, Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.), References
- Symbols used in this document:
ΔrH° Enthalpy of reaction at standard conditions - Data from NIST Standard Reference Database 69: NIST Chemistry WebBook
- The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment. However, NIST makes no warranties to that effect, and NIST shall not be liable for any damage that may result from errors or omissions in the Database.
- Customer support for NIST Standard Reference Data products.