Reaction data
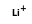 +
+ 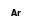 = (
= (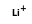 •
• 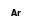 )
)
Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.)
Go To: Top, References, Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Data compiled as indicated in comments:
RCD - Robert C. Dunbar
M - Michael M. Meot-Ner (Mautner) and Sharon G. Lias
| ΔrH° (kJ/mol) | Method | Reference | Comment |
|---|---|---|---|
| 33. ± 13. | CIDT | Rodgers and Armentrout, 2000 | RCD |
| 27.0 | IMob | Gatland, 1984 | gas phase; M |
| 30. | SCATTERING | Gislason, 1984 | gas phase; M |
| 30.2 | SCATTERING | Gislason, 1984 | gas phase; M |
| 53.1 | IMob | Takebe, 1983 | gas phase; M; Data excluded from overall average |
| 17. | DT | McKnight and Sawina, 1973 | gas phase; ΔrS approximate; M; Data excluded from overall average |
References
Go To: Top, Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.), Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Rodgers and Armentrout, 2000
Rodgers, M.T.; Armentrout, P.B.,
Noncovalent Metal-Ligand Bond Energies as Studied by Threshold Collision-Induced Dissociation,
Mass Spectrom. Rev., 2000, 19, 4, 215, https://doi.org/10.1002/1098-2787(200007)19:4<215::AID-MAS2>3.0.CO;2-X
. [all data]
Gatland, 1984
Gatland, I.R.,
Swarms of Ions and Electrons in Gases, W. Lindinger, T. D. Mark and F. Howorka, eds. (Springer, New York, 1984, 1984, 44. [all data]
Gislason, 1984
Gislason, E.A.,
Quoted in I. R. Gatland in Swarms of Ions and Electrons in Gases, W. Lindinger, T. D. Mark and F. Howorka, eds. (Springer, New York, 1984, 1984, 44. [all data]
Takebe, 1983
Takebe, M.,
The Generalized Mobility Curve for Alkali Ions in Rare Gases: Clustering Reactions and Mobility Curves,
J. Chem. Phys., 1983, 78, 12, 7223, https://doi.org/10.1063/1.444763
. [all data]
McKnight and Sawina, 1973
McKnight, L.G.; Sawina, J.M.,
Equilibrium Constants and Binding Energies of Alkali Metal Ions with Inert Gases,
Bull. Am. Phys. Soc., 1973, 18, 804. [all data]
Notes
Go To: Top, Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.), References
- Symbols used in this document:
ΔrH° Enthalpy of reaction at standard conditions - Data from NIST Standard Reference Database 69: NIST Chemistry WebBook
- The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment. However, NIST makes no warranties to that effect, and NIST shall not be liable for any damage that may result from errors or omissions in the Database.
- Customer support for NIST Standard Reference Data products.