Reaction data
 +
+ 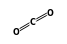 = (
= ( •
•  )
)
- Reaction by formula: O2+ + CO2 = (O2+ • CO2)
- Information on this page:
- Other data available:
- Options:
Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.)
Go To: Top, References, Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Data compiled by: Michael M. Meot-Ner (Mautner) and Sharon G. Lias
| ΔrH° (kJ/mol) | Method | Reference | Comment |
|---|---|---|---|
| 41. ± 2. | PHPMS | Hiraoka, Nakajima, et al., 1988 | gas phase; M |
| 40. ± 1. | DT | Illies, 1988 | gas phase; ΔrH(0 K)=41.0 kJ/mol; M |
| 38. ± 5.0 | PDiss | Kim, Kuo, et al., 1987 | gas phase; M |
| 43.9 | FA | Dotan, Davidson, et al., 1978 | gas phase; switching reaction(O2+)O2, Entropy change calculated or estimated; Conway and Janik, 1970; M |
| 89.5 | PHPMS | Meot-Ner (Mautner) and Field, 1977 | gas phase; Entropy change calculated or estimated, DG>, ΔrH>; M; Data excluded from overall average |
| 44.4 | PDiss | Beyer and Vanderhoff, 1976 | gas phase; ΔrH<; M; Data excluded from overall average |
References
Go To: Top, Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.), Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Hiraoka, Nakajima, et al., 1988
Hiraoka, K.; Nakajima, G.; Shoda, S.,
Determination of the Stabilities of CO2+(CO2)n and O2+(CO2)n Clusters with n = 1 - 6,
Chem. Phys. Lett., 1988, 146, 6, 535, https://doi.org/10.1016/0009-2614(88)87495-5
. [all data]
Illies, 1988
Illies, A.J.,
Thermochemistry of the Gas - Phase Ion - Molecule Clustering of CO2+CO2, SO2+CO2, N2O+N2O, O2+CO2, NO+CO2 and NO+N2O: Description of a New Hybrid Drift Tube/Ion Source with Coaxial Electron Beam and Ion Exit Apertures,
J. Phys. Chem., 1988, 92, 10, 2889, https://doi.org/10.1021/j100321a037
. [all data]
Kim, Kuo, et al., 1987
Kim, H.S.; Kuo, C.H.; Bowers, M.T.,
Photon Driven Charge Transfer Half-Collisions: The Photodissociation of CO2.O2+ Cluster Ions with Resolution of the O2 Product Vibrational States,
J. Chem. Phys., 1987, 87, 5, 2667, https://doi.org/10.1063/1.453105
. [all data]
Dotan, Davidson, et al., 1978
Dotan, I.; Davidson, J.A.; Fehsenfeld, F.C.; Albritton, D.L.,
Reactions of O2+.O2 with CO2, O3 and CH4 and O2+.O3 with H2O and CH4 and their Role in Stratospheric Ion Chemistry,
J. Geophys. Res., 1978, 83, C8, 4036, https://doi.org/10.1029/JC083iC08p04036
. [all data]
Conway and Janik, 1970
Conway, D.C.; Janik, G.S.,
Determination of the Bond Energies for the Series O2 - O2+ through O2 - O10+,
J. Chem. Phys., 1970, 53, 5, 1859, https://doi.org/10.1063/1.1674262
. [all data]
Meot-Ner (Mautner) and Field, 1977
Meot-Ner (Mautner), M.; Field, F.H.,
Proton Affinity and Ion - Molecule Clustering in CO2 and CS2. Applications in Martian Ionospheric Chemistry,
J. Chem. Phys., 1977, 66, 10, 4527, https://doi.org/10.1063/1.433706
. [all data]
Beyer and Vanderhoff, 1976
Beyer, R.A.; Vanderhoff, J.A.,
Cross Section Measurements for Photodetachment or Photodissociation of Ions Produced in Gaseous Mixtures of O2, CO2 and H2O,
J. Chem. Phys., 1976, 65, 6, 2313, https://doi.org/10.1063/1.433342
. [all data]
Notes
Go To: Top, Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.), References
- Symbols used in this document:
ΔrH° Enthalpy of reaction at standard conditions - Data from NIST Standard Reference Database 69: NIST Chemistry WebBook
- The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment. However, NIST makes no warranties to that effect, and NIST shall not be liable for any damage that may result from errors or omissions in the Database.
- Customer support for NIST Standard Reference Data products.