Trimethylsilyl, positive ion
Ion clustering data
Go To: Top, References, Notes
Data compilation copyright
by the U.S. Secretary of Commerce on behalf of the U.S.A.
All rights reserved.
Data compiled by: Michael M. Meot-Ner (Mautner) and Sharon G. Lias
Note: Please consider using the
reaction search for this species. This page allows searching
of all reactions involving this species. Searches may be limited
to ion clustering reactions. A general reaction search form is
also available.
Clustering reactions
C3H9Si+ + 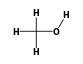 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + CH4O = (C3H9Si+ • CH4O)
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrH° | 164. | kJ/mol | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrS° | 124. | J/mol*K | N/A | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
Free energy of reaction
| ΔrG° (kJ/mol) |
T (K) |
Method |
Reference |
Comment |
| 106. | 468. | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
C3H9Si+ + 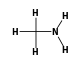 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + CH5N = (C3H9Si+ • CH5N)
C3H9Si+ + 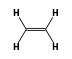 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C2H4 = (C3H9Si+ • C2H4)
C3H9Si+ + 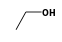 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C2H6O = (C3H9Si+ • C2H6O)
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrH° | 176. | kJ/mol | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder(CH3)3Si+))H2O, Entropy change calculated or estimated |
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrS° | 127. | J/mol*K | N/A | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder(CH3)3Si+))H2O, Entropy change calculated or estimated |
Free energy of reaction
| ΔrG° (kJ/mol) |
T (K) |
Method |
Reference |
Comment |
| 117. | 468. | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder(CH3)3Si+))H2O, Entropy change calculated or estimated |
C3H9Si+ + 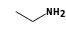 = (C3H9Si+ •
= (C3H9Si+ • 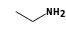 )
)
By formula: C3H9Si+ + C2H7N = (C3H9Si+ • C2H7N)
C3H9Si+ + 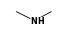 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C2H7N = (C3H9Si+ • C2H7N)
C3H9Si+ + 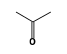 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C3H6O = (C3H9Si+ • C3H6O)
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrH° | 188. | kJ/mol | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrS° | 123. | J/mol*K | N/A | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
Free energy of reaction
| ΔrG° (kJ/mol) |
T (K) |
Method |
Reference |
Comment |
| 131. | 468. | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
C3H9Si+ + 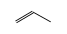 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C3H6 = (C3H9Si+ • C3H6)
C3H9Si+ + 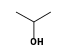 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C3H8O = (C3H9Si+ • C3H8O)
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrH° | 184. | kJ/mol | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrS° | 129. | J/mol*K | N/A | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
Free energy of reaction
| ΔrG° (kJ/mol) |
T (K) |
Method |
Reference |
Comment |
| 123. | 468. | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
C3H9Si+ + 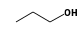 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C3H8O = (C3H9Si+ • C3H8O)
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrH° | 181. | kJ/mol | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+))H2O, Entropy change calculated or estimated |
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrS° | 129. | J/mol*K | N/A | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+))H2O, Entropy change calculated or estimated |
Free energy of reaction
| ΔrG° (kJ/mol) |
T (K) |
Method |
Reference |
Comment |
| 121. | 468. | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+))H2O, Entropy change calculated or estimated |
C3H9Si+ + 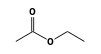 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C4H8O2 = (C3H9Si+ • C4H8O2)
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrH° | 204. | kJ/mol | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrS° | 131. | J/mol*K | N/A | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
Free energy of reaction
| ΔrG° (kJ/mol) |
T (K) |
Method |
Reference |
Comment |
| 142. | 468. | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
C3H9Si+ + 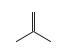 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C4H8 = (C3H9Si+ • C4H8)
C3H9Si+ + 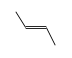 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C4H8 = (C3H9Si+ • C4H8)
C3H9Si+ + 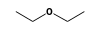 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C4H10O = (C3H9Si+ • C4H10O)
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrH° | 185. | kJ/mol | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrS° | 125. | J/mol*K | N/A | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
Free energy of reaction
| ΔrG° (kJ/mol) |
T (K) |
Method |
Reference |
Comment |
| 127. | 468. | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
C3H9Si+ +  = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C4H10O = (C3H9Si+ • C4H10O)
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrH° | 185. | kJ/mol | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrS° | 130. | J/mol*K | N/A | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
Free energy of reaction
| ΔrG° (kJ/mol) |
T (K) |
Method |
Reference |
Comment |
| 124. | 468. | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
C3H9Si+ + 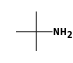 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C4H11N = (C3H9Si+ • C4H11N)
C3H9Si+ +  = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C4H11N = (C3H9Si+ • C4H11N)
C3H9Si+ + 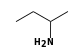 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C4H11N = (C3H9Si+ • C4H11N)
C3H9Si+ + 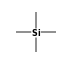 = (C3H9Si+ •
= (C3H9Si+ • 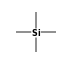 )
)
By formula: C3H9Si+ + C4H12Si = (C3H9Si+ • C4H12Si)
C3H9Si+ +  = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C5H10O2 = (C3H9Si+ • C5H10O2)
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrH° | 210. | kJ/mol | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrS° | 132. | J/mol*K | N/A | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
Free energy of reaction
| ΔrG° (kJ/mol) |
T (K) |
Method |
Reference |
Comment |
| 149. | 468. | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
C3H9Si+ + 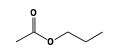 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C5H10O2 = (C3H9Si+ • C5H10O2)
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrH° | 207. | kJ/mol | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrS° | 132. | J/mol*K | N/A | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
Free energy of reaction
| ΔrG° (kJ/mol) |
T (K) |
Method |
Reference |
Comment |
| 145. | 468. | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
C3H9Si+ + 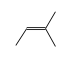 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C5H10 = (C3H9Si+ • C5H10)
C3H9Si+ + 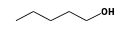 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C5H12O = (C3H9Si+ • C5H12O)
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrH° | 187. | kJ/mol | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrS° | 131. | J/mol*K | N/A | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
Free energy of reaction
| ΔrG° (kJ/mol) |
T (K) |
Method |
Reference |
Comment |
| 126. | 468. | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
C3H9Si+ + 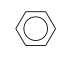 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C6H6 = (C3H9Si+ • C6H6)
C3H9Si+ + 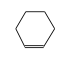 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C6H10 = (C3H9Si+ • C6H10)
C3H9Si+ + 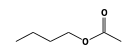 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C6H12O2 = (C3H9Si+ • C6H12O2)
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrH° | 209. | kJ/mol | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrS° | 133. | J/mol*K | N/A | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
Free energy of reaction
| ΔrG° (kJ/mol) |
T (K) |
Method |
Reference |
Comment |
| 147. | 468. | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
C3H9Si+ + 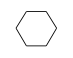 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C6H12 = (C3H9Si+ • C6H12)
C3H9Si+ + 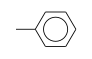 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C7H8 = (C3H9Si+ • C7H8)
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrH° | 119. | kJ/mol | PHPMS | Stone and Stone, 1991 | gas phase; forms pi complex |
| ΔrH° | 131. | kJ/mol | PHPMS | Stone and Stone, 1991 | gas phase; toluene D8, forms pi complex |
| ΔrH° | 111. | kJ/mol | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)C6H6, Entropy change calculated or estimated |
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrS° | 146. | J/mol*K | N/A | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)C6H6, Entropy change calculated or estimated |
Free energy of reaction
| ΔrG° (kJ/mol) |
T (K) |
Method |
Reference |
Comment |
| 43.1 | 468. | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)C6H6, Entropy change calculated or estimated |
C3H9Si+ + 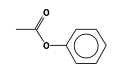 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C8H8O2 = (C3H9Si+ • C8H8O2)
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrH° | 204. | kJ/mol | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrS° | 133. | J/mol*K | N/A | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
Free energy of reaction
| ΔrG° (kJ/mol) |
T (K) |
Method |
Reference |
Comment |
| 141. | 468. | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
C3H9Si+ + 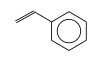 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C8H8 = (C3H9Si+ • C8H8)
C3H9Si+ + 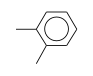 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C8H10 = (C3H9Si+ • C8H10)
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrH° | 122. | kJ/mol | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)C6H6, Entropy change calculated or estimated |
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrS° | 147. | J/mol*K | N/A | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)C6H6, Entropy change calculated or estimated |
Free energy of reaction
| ΔrG° (kJ/mol) |
T (K) |
Method |
Reference |
Comment |
| 53.1 | 468. | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)C6H6, Entropy change calculated or estimated |
C3H9Si+ + 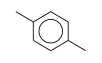 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C8H10 = (C3H9Si+ • C8H10)
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrH° | 118. | kJ/mol | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)C6H6, Entropy change calculated or estimated |
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrS° | 147. | J/mol*K | N/A | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)C6H6, Entropy change calculated or estimated |
Free energy of reaction
| ΔrG° (kJ/mol) |
T (K) |
Method |
Reference |
Comment |
| 49.4 | 468. | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)C6H6, Entropy change calculated or estimated |
C3H9Si+ + 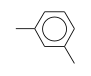 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C8H10 = (C3H9Si+ • C8H10)
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrH° | 121. | kJ/mol | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)C6H6, Entropy change calculated or estimated |
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrS° | 147. | J/mol*K | N/A | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)C6H6, Entropy change calculated or estimated |
Free energy of reaction
| ΔrG° (kJ/mol) |
T (K) |
Method |
Reference |
Comment |
| 53.1 | 468. | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)C6H6, Entropy change calculated or estimated |
C3H9Si+ + 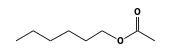 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C8H16O2 = (C3H9Si+ • C8H16O2)
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrH° | 211. | kJ/mol | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrS° | 134. | J/mol*K | N/A | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
Free energy of reaction
| ΔrG° (kJ/mol) |
T (K) |
Method |
Reference |
Comment |
| 149. | 468. | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
C3H9Si+ + 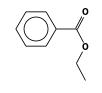 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C9H10O2 = (C3H9Si+ • C9H10O2)
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrH° | 213. | kJ/mol | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrS° | 134. | J/mol*K | N/A | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
Free energy of reaction
| ΔrG° (kJ/mol) |
T (K) |
Method |
Reference |
Comment |
| 150. | 468. | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)H2O, Entropy change calculated or estimated |
C3H9Si+ + 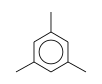 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + C9H12 = (C3H9Si+ • C9H12)
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrH° | 130. | kJ/mol | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)(C6H6), Entropy change calculated or estimated |
| Quantity |
Value |
Units |
Method |
Reference |
Comment |
| ΔrS° | 147. | J/mol*K | N/A | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)(C6H6), Entropy change calculated or estimated |
Free energy of reaction
| ΔrG° (kJ/mol) |
T (K) |
Method |
Reference |
Comment |
| 61.1 | 468. | PHPMS | Wojtyniak and Stone, 1986 | gas phase; switching reaction,Thermochemical ladder((CH3)3Si+)(C6H6), Entropy change calculated or estimated |
C3H9Si+ + 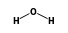 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + H2O = (C3H9Si+ • H2O)
(C3H9Si+ •  ) +
) +  = (C3H9Si+ • 2
= (C3H9Si+ • 2 )
)
By formula: (C3H9Si+ • H2O) + H2O = (C3H9Si+ • 2H2O)
C3H9Si+ + 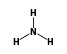 = (C3H9Si+ •
= (C3H9Si+ •  )
)
By formula: C3H9Si+ + H3N = (C3H9Si+ • H3N)
References
Go To: Top, Ion clustering data, Notes
Data compilation copyright
by the U.S. Secretary of Commerce on behalf of the U.S.A.
All rights reserved.
Wojtyniak and Stone, 1986
Wojtyniak, A.C.M.; Stone, A.J.,
A High-Pressure Mass Spectrometric Study of the Bonding of Trimethylsilylium to Oxygen and Aromatic Bases,
Can. J. Chem., 1986, 74, 59. [all data]
Li and Stone, 1990
Li, X.; Stone, A.J.,
Gas-Phase (CH3)3Si+ Affinities of Alkylamines and Proton Affinities of Trimethylsilyl Alkylamines,
Int. J. Mass Spectrom. Ion Proc., 1990, 101, 2-3, 149, https://doi.org/10.1016/0168-1176(90)87008-5
. [all data]
Li and Stone, 1989
Li, X.; Stone, J.A.,
Determination of the beta silicon effect from a mass spectrometric study of the association of trimethylsilylium ion with alkenes,
J. Am. Chem. Soc., 1989, 111, 15, 5586, https://doi.org/10.1021/ja00197a013
. [all data]
Wojtyniak, Li, et al., 1987
Wojtyniak, A.C.M.; Li, K.; Stone, J.A.,
The Formation of (CH3)7Si2+ in (CH3)4Si/CH4 Mixtures and CH3- Exchange Reactions Between (CH3)4Si, (CH3)4Ge and (CH3)4Sn Studied by High Pressure Mass Spectrometry,
Can. J. Chem., 1987, 65, 12, 2849, https://doi.org/10.1139/v87-473
. [all data]
Stone and Stone, 1991
Stone, J.M.; Stone, J.A.,
A High Pressure Mass Spectrometric Study of the Binding of (CH3)3Si+ and (CH3)3C+ to Toluene and Benzene,
Int. J. Mass Spectrom. Ion Proc., 1991, 109, 247, https://doi.org/10.1016/0168-1176(91)85107-W
. [all data]
Stone and Wojtyniak, 1986
Stone, A.J.; Wojtyniak, A.C.M.,
The Condensation of Trimethylsilylium with Water and the Proton Affinity of Trimethylsilanol,
Can. J. Chem., 1986, 64, 3, 575, https://doi.org/10.1139/v86-092
. [all data]
Stone and Wytenberg, 1987
Stone, J.A.; Wytenberg, W.J.,
The Binding Energies of Trialkylgermanium Cations to Water Molecules Studied by High Pressure Mass Spectrometry,
Can. J. Chem., 1987, 65, 9, 2146, https://doi.org/10.1139/v87-358
. [all data]
Notes
Go To: Top, Ion clustering data, References
- Symbols used in this document:
| T |
Temperature |
| ΔrG° |
Free energy of reaction at standard conditions |
| ΔrH° |
Enthalpy of reaction at standard conditions |
| ΔrS° |
Entropy of reaction at standard conditions |
- Data from NIST Standard Reference Database 69:
NIST Chemistry WebBook
-
The National Institute of Standards and Technology (NIST)
uses its best efforts to deliver a high quality copy of the
Database and to verify that the data contained therein have
been selected on the basis of sound scientific judgment.
However, NIST makes no warranties to that effect, and NIST
shall not be liable for any damage that may result from
errors or omissions in the Database.
-
Customer support
for NIST Standard Reference Data products.
) +
= (C3H9Si+ • 2
)