Reaction data
(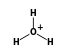 • 3
• 3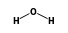 ) +
) +  = (
= ( • 4
• 4 )
)
- Reaction by formula: (H3O+ • 3H2O) + H2O = (H3O+ • 4H2O)
- Comments:
- Bond type: Hydrogen bond (positive ion to hydride)
- Information on this page:
- Other data available:
- Options:
Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.)
Go To: Top, References, Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Data compiled by: Michael M. Meot-Ner (Mautner) and Sharon G. Lias
| ΔrH° (kJ/mol) | Method | Reference | Comment |
|---|---|---|---|
| 53.1 ± 5.9 | CID | Dalleska, Honma, et al., 1993 | gas phase; guided ion beam CID; M |
| 48.1 | PHPMS | Meot-Ner (Mautner) and Speller, 1986 | gas phase; M |
| 53.1 | PHPMS | Lau, Ikuta, et al., 1982 | gas phase; M |
| 53.1 | HPMS | Arifov, Pozharov, et al., 1973 | gas phase; M |
| 72.8 | PHPMS | Tholman, Tonner, et al., 1994 | gas phase; M |
| 54.0 | HPMS | Beggs and Field, 1971 | gas phase; M |
| 34. | CID | Magnera, David, et al., 1991 | gas phase; M; Data excluded from overall average |
| 61.9 | HPMS | Field, 1969 | gas phase; M; Data excluded from overall average |
| 64.0 | HPMS | Kebarle, Searles, et al., 1967 | gas phase; M; Data excluded from overall average |
References
Go To: Top, Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.), Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Dalleska, Honma, et al., 1993
Dalleska, N.F.; Honma, K.; Armentrout, P.B.,
Stepwise Solvation Enthalpies of Protonated Water Clusters: Collision-Induced Dissociation as an Aleternative to Equilibrium Studies,
J. Am. Chem. Soc., 1993, 115, 25, 12125, https://doi.org/10.1021/ja00078a059
. [all data]
Meot-Ner (Mautner) and Speller, 1986
Meot-Ner (Mautner), M.; Speller, C.V.,
The Filling of Solvent Shells in Cluster Ions: Thermochemical Criteria nd the Effects of Isomeric Clusters,
J. Phys. Chem., 1986, 90, 25, 6616, https://doi.org/10.1021/j100283a006
. [all data]
Lau, Ikuta, et al., 1982
Lau, Y.K.; Ikuta, S.; Kebarle, P.,
Thermodynamics and Kinetics of the Gas - Phase Reactions: H3O+(H2O)n - 1 + H2O = H3O+(H2O)n,
J. Am. Chem. Soc., 1982, 104, 6, 1462, https://doi.org/10.1021/ja00370a002
. [all data]
Arifov, Pozharov, et al., 1973
Arifov, U.A.; Pozharov, S.L.; Chernov, I.G.; Mukhamediev, Z.A.,
High Energy Chem., 1973, 7, 347. [all data]
Tholman, Tonner, et al., 1994
Tholman, D.; Tonner, D.S.; McMahon, T.B.,
Spontaneous Unimolecular Dissociation of Small Cluster Ions, (H3O)+(L)n and Cl-(H2O)n (n = 2-4), under Fourier Transform Ion Cyclotron Resonance Conditions,
J. Phys. Chem., 1994, 98, 8, 2002, https://doi.org/10.1021/j100059a002
. [all data]
Beggs and Field, 1971
Beggs, D.P.; Field, F.H.,
Reversible Reactions of Gaseous Ions. I. Methane-Water System,
J. Am. Chem. Soc., 1971, 93, 7, 1567, https://doi.org/10.1021/ja00736a001
. [all data]
Magnera, David, et al., 1991
Magnera, T.F.; David, D.E.; Michl, J.,
The First Twenty - Eight Proton Hydration Energies,
Chem. Phys. Lett., 1991, 182, 3-4, 363, https://doi.org/10.1016/0009-2614(91)80230-U
. [all data]
Field, 1969
Field, F.H.,
Chemical Ionization Mass Spectrometry. IX. Temperature and Pressure Studies with Benzyl Acetate and t-Amyl Acetate,
J. Am. Chem. Soc., 1969, 91, 11, 2827, https://doi.org/10.1021/ja01039a002
. [all data]
Kebarle, Searles, et al., 1967
Kebarle, P.; Searles, S.K.; Zolla, A.; Scarborough, J.; Arshadi, M.,
The Solvation of the Hydrogen Ion by Water Molecules in the Gas Phase. Heats and Entropies of Solvation of the Individual Reactions: H+(H2O)n-1 + H2O = H+(H2O)n,
J. Am. Chem. Soc., 1967, 89, 25, 6393, https://doi.org/10.1021/ja01001a001
. [all data]
Notes
Go To: Top, Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.), References
- Symbols used in this document:
ΔrH° Enthalpy of reaction at standard conditions - Data from NIST Standard Reference Database 69: NIST Chemistry WebBook
- The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment. However, NIST makes no warranties to that effect, and NIST shall not be liable for any damage that may result from errors or omissions in the Database.
- Customer support for NIST Standard Reference Data products.