Reaction data
H3+ + 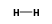 = (H3+ •
= (H3+ • 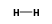 )
)
Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.)
Go To: Top, References, Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Data compiled by: Michael M. Meot-Ner (Mautner) and Sharon G. Lias
| ΔrH° (kcal/mol) | Method | Reference | Comment |
|---|---|---|---|
| 7.0 ± 0.1 | PHPMS | Hiraoka and Mori, 1989 | gas phase; M |
| 6.9 ± 0.3 | PHPMS | Hiraoka, 1987 | gas phase; M |
| 6.6 | HPMS | Beuhler, Ehrenson, et al., 1983 | gas phase; Entropy change is questionable; M |
| 7.1 | HPMS | Beuhler, Ehrenson, et al., 1983 | gas phase; deuterated; M |
| 5.8 | DT | Elford, 1983 | gas phase; Entropy change is questionable; M; Data excluded from overall average |
| 5.8 ± 1.1 | IMob | Elford, 1983 | gas phase; M; Data excluded from overall average |
| 8.6 | DT | Johnsen, Huang, et al., 1976 | gas phase; corrected for ln T term by Keesee and Castleman, 1986; M; Data excluded from overall average |
| 9.6 | PHPMS | Hiraoka and Kebarle, 1975 | gas phase; M; Data excluded from overall average |
| 9.7 | HPMS | Bennett and Field, 1972 | gas phase; M; Data excluded from overall average |
| 5.1 | HPMS | Arifov, Pozharov, et al., 1971 | gas phase; Entropy change is questionable; M; Data excluded from overall average |
| 5.6 | HPMS | Arifov, Pozharov, et al., 1971, 2 | gas phase; deuterated, Entropy change is questionable; M; Data excluded from overall average |
References
Go To: Top, Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.), Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Hiraoka and Mori, 1989
Hiraoka, K.; Mori, T.,
Isotope Effect and Nature of Bonding in the Cluster Ions H3+(Ar)n and D3+(Ar)n,
J. Chem. Phys., 1989, 91, 8, 4821, https://doi.org/10.1063/1.456720
. [all data]
Hiraoka, 1987
Hiraoka, K.,
A Determination of the Stabilities of H3+(H2)n with n=1-9 from Measurements of the gas-Phase Ion Equilibria H3+(H2)n-1 + H2 = H3+(H2)n,
J. Chem. Phys., 1987, 87, 7, 4048, https://doi.org/10.1063/1.452909
. [all data]
Beuhler, Ehrenson, et al., 1983
Beuhler, R.J.; Ehrenson, S.; Friedman, L.,
Hydrogen Cluster Ion Equilibria,
J. Chem. Phys., 1983, 79, 12, 5982, https://doi.org/10.1063/1.445781
. [all data]
Elford, 1983
Elford, M.T.,
The Heat of Dissociation of H5+ Derived from Measurements of Ion Mobilities,
J. Chem. Phys., 1983, 79, 12, 5951, https://doi.org/10.1063/1.445777
. [all data]
Johnsen, Huang, et al., 1976
Johnsen, R.; Huang, C.M.; Biondi, M.A.,
Three - Body Association Reactions of H+ and H3+ Ions in Hydrogen from 135 to 300K,
J. Chem. Phys., 1976, 65, 4, 1539, https://doi.org/10.1063/1.433209
. [all data]
Keesee and Castleman, 1986
Keesee, R.G.; Castleman, A.W., Jr.,
Thermochemical data on Ggs-phase ion-molecule association and clustering reactions,
J. Phys. Chem. Ref. Data, 1986, 15, 1011. [all data]
Hiraoka and Kebarle, 1975
Hiraoka, K.; Kebarle, P.,
A Determination of the Stabilities of H5+, H7+, H9+, and H11+ from Measurement of the Gas Phase Ion Equilibria Hn+ + H2 = H(n + 2)+ (n = 3, 5, 7, 9),
J. Chem. Phys., 1975, 62, 6, 2267, https://doi.org/10.1063/1.430751
. [all data]
Bennett and Field, 1972
Bennett, S.L.; Field, F.H.,
Reversible Reactions of Gaseous Ions. VII. The Hydrogen System,
J. Am. Chem. Soc., 1972, 94, 25, 8669, https://doi.org/10.1021/ja00780a003
. [all data]
Arifov, Pozharov, et al., 1971
Arifov, U.A.; Pozharov, S.L.; Chernov, I.G.; Mukhamediev, Z.A.,
Ionic-Molecular Reactions in Hydrogen at High Pressures,
High Energy Chem., 1971, 5, 69. [all data]
Arifov, Pozharov, et al., 1971, 2
Arifov, U.A.; Pozharov, S.L.; Chernov, I.G.; Mukhamediev, Z.A.,
Mass Spectrometric Study of the Kinetics of D5+ Formation,
High Energy Chem., 1971, 5, 79. [all data]
Notes
Go To: Top, Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.), References
- Symbols used in this document:
ΔrH° Enthalpy of reaction at standard conditions - Data from NIST Standard Reference Database 69: NIST Chemistry WebBook
- The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment. However, NIST makes no warranties to that effect, and NIST shall not be liable for any damage that may result from errors or omissions in the Database.
- Customer support for NIST Standard Reference Data products.