Reaction data
C2H- + 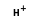 =
= 
Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.)
Go To: Top, References, Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Data compiled by: John E. Bartmess
| ΔrH° (kcal/mol) | Method | Reference | Comment |
|---|---|---|---|
| 378.460 ± 0.070 | D-EA | Zhou, Garand, et al., 2007 | gas phase; B |
| 378.45 ± 0.15 | D-EA | Ervin and Lineberger, 1991 | gas phase; B |
| 377.97 ± 0.67 | G+TS | Ervin, Gronert, et al., 1990 | gas phase; B |
| 378.00 ± 0.50 | N/A | Ruscic and Berkowitz, 1990 | gas phase; 376.7 0K; corrected with data from Gurvich, Veyts, et al.; B |
| 379.80 ± 0.50 | TDEq | Meot-ner and Sieck, 1986 | gas phase; value altered from reference due to change in acidity scale; B |
| 376.7 ± 2.1 | G+TS | Bartmess, Scott, et al., 1979 | gas phase; value altered from reference due to change in acidity scale; B |
| 384.97 ± 0.67 | G+TS | Bohme, MacKay, et al., 1974 | gas phase; B |
| 375.8 ± 9.2 | Endo | Hughes, Lifschitz, et al., 1973 | gas phase; B |
References
Go To: Top, Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.), Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Zhou, Garand, et al., 2007
Zhou, J.; Garand, E.; Neumark, D.M.,
Vibronic structure in C2H and C2D from anion slow electron velocity-map imaging spectroscopy,
J. Chem. Phys., 2007, 127, 11, 114313, https://doi.org/10.1063/1.2768932
. [all data]
Ervin and Lineberger, 1991
Ervin, K.M.; Lineberger, W.C.,
Photoelectron Spectra of C2- and C2H-,
J. Phys. Chem., 1991, 95, 3, 1167, https://doi.org/10.1021/j100156a026
. [all data]
Ervin, Gronert, et al., 1990
Ervin, K.M.; Gronert, S.; Barlow, S.E.; Gilles, M.K.; Harrison, A.G.; Bierbaum, V.M.; DePuy, C.H.; Lin, W.C.,
Bonds Strengths of Ethylene and Acetylene,
J. Am. Chem. Soc., 1990, 112, 15, 5750, https://doi.org/10.1021/ja00171a013
. [all data]
Ruscic and Berkowitz, 1990
Ruscic, B.; Berkowitz, J.,
Photoion-pair Formation and Photoelectron-induced Dissociative Attachment in C2H2: D0(HCC-H),
J. Chem. Phys., 1990, 93, 8, 5586, https://doi.org/10.1063/1.459629
. [all data]
Gurvich, Veyts, et al.
Gurvich, L.V.; Veyts, I.V.; Alcock, C.B.,
Hemisphere Publishing, NY, 1989, V. 1 2, Thermodynamic Properties of Individual Substances, 4th Ed. [all data]
Meot-ner and Sieck, 1986
Meot-ner, M.; Sieck, L.W.,
Relative acidities of water and methanol, and the stabilities of the dimer adducts,
J. Phys. Chem., 1986, 90, 6687. [all data]
Bartmess, Scott, et al., 1979
Bartmess, J.E.; Scott, J.A.; McIver, R.T., Jr.,
The gas phase acidity scale from methanol to phenol,
J. Am. Chem. Soc., 1979, 101, 6047. [all data]
Bohme, MacKay, et al., 1974
Bohme, D.K.; MacKay, G.I.; Schiff, H.I.; Hemsworth, R.S.,
Equilibrium OH- + C2H2 = C2H- + H2O and the determination of Hf298(C2H-),
J. Chem. Phys., 1974, 61, 2175. [all data]
Hughes, Lifschitz, et al., 1973
Hughes, B.M.; Lifschitz, C.; Tiernan, T.O.,
Electron affinities from endothermic negative-ion charge-transfer reactions. III. NO, NO2, S2, CS2, Cl2, Br2, I2, and C2H,
J. Chem. Phys., 1973, 59, 3162. [all data]
Notes
Go To: Top, Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.), References
- Symbols used in this document:
ΔrH° Enthalpy of reaction at standard conditions - Data from NIST Standard Reference Database 69: NIST Chemistry WebBook
- The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment. However, NIST makes no warranties to that effect, and NIST shall not be liable for any damage that may result from errors or omissions in the Database.
- Customer support for NIST Standard Reference Data products.