Reaction data
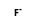 +
+ 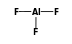 = (
= (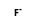 •
•  )
)
- Reaction by formula: F- + AlF3 = (F- • AlF3)
- Information on this page:
- Other data available:
- Options:
Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.)
Go To: Top, References, Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Data compiled as indicated in comments:
B - John E. Bartmess
M - Michael M. Meot-Ner (Mautner) and Sharon G. Lias
| ΔrH° (kcal/mol) | Method | Reference | Comment |
|---|---|---|---|
| 116.6 ± 1.9 | TDAs | Nikitin, Igolkina, et al., 1986 | gas phase; Summary of literature data plus new work. Recommended average value at 0K.; value altered from reference due to conversion from electron convention to ion convention; B |
| 119.0 ± 1.6 | TDAs | Sidorov, Nikitin, et al., 1980 | gas phase; Fluoride Affinity:1100K ΔHf(AlF4-):298K; value altered from reference due to conversion from electron convention to ion convention; B |
| 119.5 ± 2.0 | TDEq | Nikitin, Sidorov, et al., 1981 | gas phase; Fluoride Affinity: 4.1 kcal > ScF3; value altered from reference due to conversion from electron convention to ion convention; B |
| 118.5 ± 3.0 | TDEq | Gusarov, Pyatenko, et al., 1980 | gas phase; KF2- + KAlF4 <=> AlF4- + 2KF; value altered from reference due to conversion from electron convention to ion convention; B |
| 118.2 ± 2.7 | TDEq | Nikitin, Skokan, et al., 1979 | gas phase; Fluoride Affinity: 22.2±.2 kcal > UF4; value altered from reference due to conversion from electron convention to ion convention; B,M |
| 114. | MS | Pyatenko, Gusarov, et al., 1981 | gas phase; Knudsen cell; M |
| 150. ± 10. | Ther | Srivastava, Uy, et al., 1974 | gas phase; 2 AlF2 + AlF2- <=> 2AlF + AlF4-; B |
References
Go To: Top, Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.), Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Nikitin, Igolkina, et al., 1986
Nikitin, M.I.; Igolkina, N.A.; Skokan, E.V.; Sorokin, I.D.; Sidirov, L.N.,
Enthalpies of formation of the AlF4- ion,
J. Phys. Chem., 1986, 60, 22. [all data]
Sidorov, Nikitin, et al., 1980
Sidorov, L.N.; Nikitin, M.I.; Skokan, E.V.; Sorokin, I.D.,
Mass-spectrometric determination of enthalpies of dissociation of gaseous complex fluorides into neutral and charged particles. II. Heats of formation of AlF4- and KF2-,
Int. J. Mass Spectrom. Ion Phys., 1980, 35, 203. [all data]
Nikitin, Sidorov, et al., 1981
Nikitin, M.I.; Sidorov, L.N.; Skokan, E.V.; Sorokin, I.D.,
Mass spectrometric determination of the heats of formation of ScF4- and KF2-,
Russ. J. Phys. Chem., 1981, 55, 1107. [all data]
Gusarov, Pyatenko, et al., 1980
Gusarov, A.V.; Pyatenko, A.T.; Gorokhov, L.N.,
Tetrafluoroaluminate (AlF4-) and Fluoroaluminate (Al2F7-) Ions in Aluminum Fluoride Vapors,
Teplofiz. Vys. Temperatiur., 1980, 18, 10584y. [all data]
Nikitin, Skokan, et al., 1979
Nikitin, M.I.; Skokan, E.V.; Sorokin, I.D.; Sidorov, L.N.,
Negative Ions in Saturated Vapor of the System MF-AlF3. Heat of Formation of AlF4-,
Dokl. Akad. Nauk SSSR Ser. Khim., 1979, 247, 151. [all data]
Pyatenko, Gusarov, et al., 1981
Pyatenko, A.T.; Gusarov, A.V.; Gorokhov, L.N.,
Negative Ions in the Vapor Over Lanthanum Trifluoride,
High Temp., 1981, 19, 241. [all data]
Srivastava, Uy, et al., 1974
Srivastava, R.D.; Uy, O.M.; Farber, M.,
Experimental determination of heats of formation of negative ions and electron affinities of several boron and aluminum fluorides,
J. Chem. Soc. Faraday Trans. 1, 1974, 70, 1033. [all data]
Notes
Go To: Top, Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.), References
- Symbols used in this document:
ΔrH° Enthalpy of reaction at standard conditions - Data from NIST Standard Reference Database 69: NIST Chemistry WebBook
- The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment. However, NIST makes no warranties to that effect, and NIST shall not be liable for any damage that may result from errors or omissions in the Database.
- Customer support for NIST Standard Reference Data products.