Reaction data
(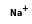 •
• 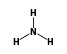 ) +
) +  = (
= (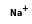 • 2
• 2 )
)
- Reaction by formula: (Na+ • H3N) + H3N = (Na+ • 2H3N)
- Information on this page:
- Other data available:
- Options:
Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.)
Go To: Top, References, Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Data compiled as indicated in comments:
RCD - Robert C. Dunbar
M - Michael M. Meot-Ner (Mautner) and Sharon G. Lias
| ΔrH° (kJ/mol) | Method | Reference | Comment |
|---|---|---|---|
| 95.4 ± 4.6 | CIDC | Amicangelo and Armentrout, 2001 | Anchor NH3=24.41; RCD |
| 89.5 ± 4.6 | CIDC | Amicangelo and Armentrout, 2001 | Anchor NH3=24.41; RCD |
| 95.4 ± 6.7 | CIDC | Amicangelo and Armentrout, 2001 | Anchor NH3=24.41; RCD |
| 89.5 ± 5.9 | CIDC | Amicangelo and Armentrout, 2001 | Anchor NH3=24.41; RCD |
| 91.6 ± 5.9 | CIDC | Amicangelo and Armentrout, 2001 | Anchor NH3=24.41; RCD |
| 95.8 | HPMS | Castleman, Holland, et al., 1978 | gas phase; M |
References
Go To: Top, Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.), Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Amicangelo and Armentrout, 2001
Amicangelo, J.C.; Armentrout, P.B.,
Relative and Absolute Bond Dissociation Energies of Sodium Cation Complexes Determined Using Competitive Collision-Induced Dissociation Experiments,
Int. J. Mass Spectrom., 2001, 212, 1-3, 301, https://doi.org/10.1016/S1387-3806(01)00494-8
. [all data]
Castleman, Holland, et al., 1978
Castleman, A.W.; Holland, P.M.; Lindsay, D.M.; Peterson, K.I.,
The Properties of Clusters in the Gas Phase. 2. Ammonia about Metal Ions,
J. Am. Chem. Soc., 1978, 100, 19, 6039, https://doi.org/10.1021/ja00487a011
. [all data]
Notes
Go To: Top, Enthalpy of reaction at standard conditions (nominally 298.15 K, 1 atm.), References
- Symbols used in this document:
ΔrH° Enthalpy of reaction at standard conditions - Data from NIST Standard Reference Database 69: NIST Chemistry WebBook
- The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment. However, NIST makes no warranties to that effect, and NIST shall not be liable for any damage that may result from errors or omissions in the Database.
- Customer support for NIST Standard Reference Data products.