Reaction data
(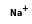 • 3
• 3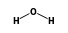 ) +
) +  = (
= (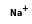 • 4
• 4 )
)
- Reaction by formula: (Na+ • 3H2O) + H2O = (Na+ • 4H2O)
- Information on this page:
- Other data available:
- Options:
Entropy of reaction at standard conditions (nominally 298.15 K, 1 atm.)
Go To: Top, References, Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Data compiled by: Michael M. Meot-Ner (Mautner) and Sharon G. Lias
| ΔrS° (J/mol*K) | Method | Reference | Comment |
|---|---|---|---|
| 92.0 | HPMS | Castleman, Holland, et al., 1978 | gas phase; M |
| 99.2 | HPMS | Tang, Lian, et al., 1976 | gas phase; M |
| 109. | HPMS | Tang and Castleman, 1972 | gas phase; M |
| 105. | HPMS | Dzidic and Kebarle, 1970 | gas phase; M |
| 92.0 | ES/HPMS | Blades, Klassen, et al., 1996 | gas phase; M |
| 96. | N/A | Blades, Jayaweera, et al., 1990 | gas phase; electrospray, Entropy change calculated or estimated; M |
References
Go To: Top, Entropy of reaction at standard conditions (nominally 298.15 K, 1 atm.), Notes
Data compilation copyright by the U.S. Secretary of Commerce on behalf of the U.S.A. All rights reserved.
Castleman, Holland, et al., 1978
Castleman, A.W.; Holland, P.M.; Lindsay, D.M.; Peterson, K.I.,
The Properties of Clusters in the Gas Phase. 2. Ammonia about Metal Ions,
J. Am. Chem. Soc., 1978, 100, 19, 6039, https://doi.org/10.1021/ja00487a011
. [all data]
Tang, Lian, et al., 1976
Tang, I.N.; Lian, M.S.; Castleman, A.W.,
Mass Spectrometric Study of Gas - Phase Clustering Reactions: Hydration of the Monovalent Strontium Ion,
J. Chem. Phys., 1976, 65, 10, 4022, https://doi.org/10.1063/1.432854
. [all data]
Tang and Castleman, 1972
Tang, I.N.; Castleman, A.W.,
Mass Spectrometric Study of the Gas - Phase Hydration of the Monovalent Lead Ion,
J. Chem. Phys., 1972, 57, 9, 3638, https://doi.org/10.1063/1.1678820
. [all data]
Dzidic and Kebarle, 1970
Dzidic, I.; Kebarle, P.,
Hydration of the Alkali Ions in the Gas Phase. Enthalpies and Entropies of Reactions M+(H2O)n-1 + H2O = M+(H2O)n,
J. Phys. Chem., 1970, 74, 7, 1466, https://doi.org/10.1021/j100702a013
. [all data]
Blades, Klassen, et al., 1996
Blades, A.T.; Klassen, J.S.; Kebarle, P.,
Determination of Ion-Solvent Equilibria in the Gas Phase. Hydration of Diprotonated Diamines and Bis(trimethylammonium) Alkanes,
J. Am. Chem. Soc., 1996, 118, 49, 12437, https://doi.org/10.1021/ja962641t
. [all data]
Blades, Jayaweera, et al., 1990
Blades, A.T.; Jayaweera, P.; Ikonomou, M.G.; Kebarle, P.,
Studies of Alkaline - Earth and Transition - Metal M++ Gas - Phase Ion Chemistry,
J. Chem. Phys., 1990, 92, 10, 5900, https://doi.org/10.1063/1.458360
. [all data]
Notes
Go To: Top, Entropy of reaction at standard conditions (nominally 298.15 K, 1 atm.), References
- Symbols used in this document:
ΔrS° Entropy of reaction at standard conditions - Data from NIST Standard Reference Database 69: NIST Chemistry WebBook
- The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high quality copy of the Database and to verify that the data contained therein have been selected on the basis of sound scientific judgment. However, NIST makes no warranties to that effect, and NIST shall not be liable for any damage that may result from errors or omissions in the Database.
- Customer support for NIST Standard Reference Data products.